ISIIS-DPI Technology
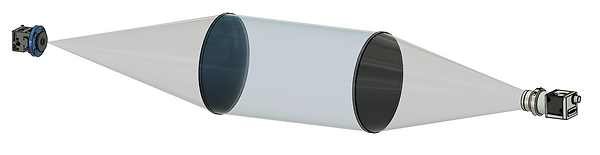
The ISIIS-DPI imaging systems uses a shadowgraph technique. Light from an LED source passes through a plano-convex lens, creating a collimated beam of light through the volume of sampled water. The shadow of the organisms is then refocused and projected onto a camera. The advantages of this approach include high depth of field, telecentric image (magnification level not affected by distance from object to the lens), and very sharp outlines of organisms and internal structures which facilitates automated recognition.
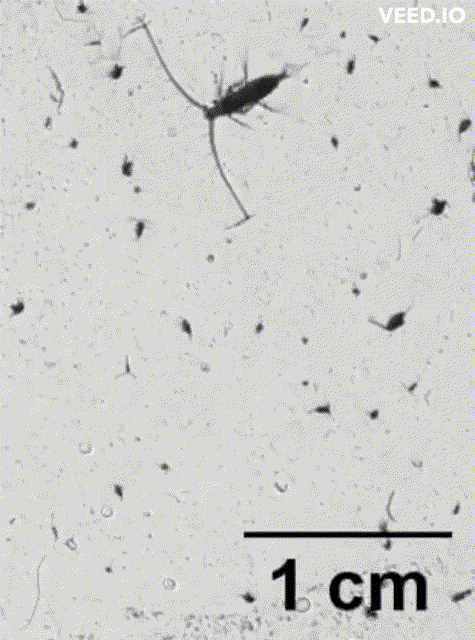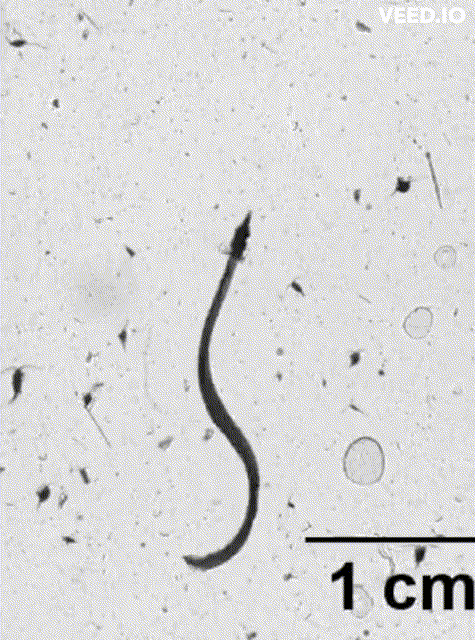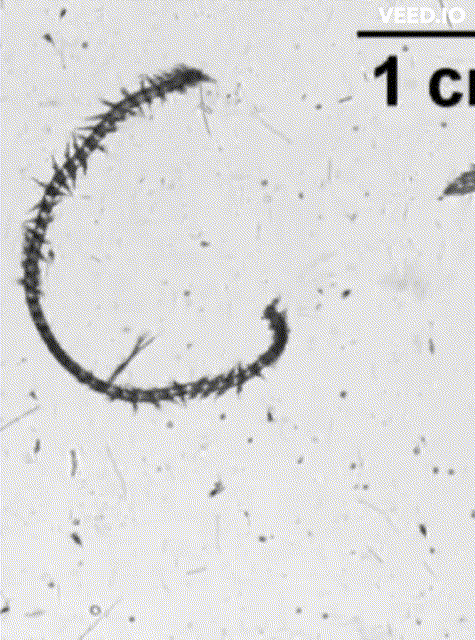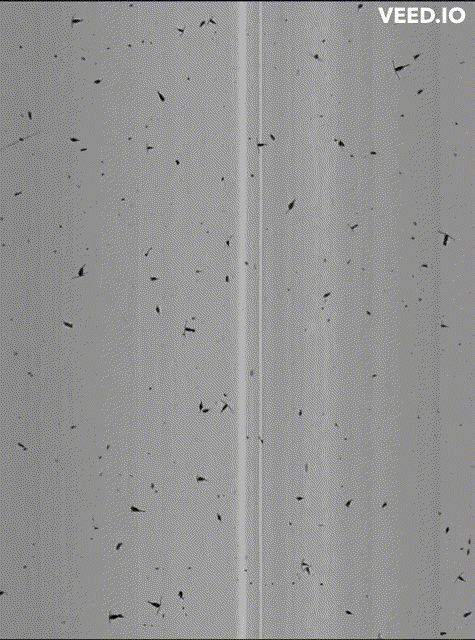
credit: Dr. Adam Greer, SKIO

FAQ
What is it?
ISIIS plankton imagers are based on a very specific back-illumination imaging technique. It is a focused shadowgraph system that captures silhouettes of plankton. It uses a pseudo collimated beam of light ensuring projections/shadows of organisms that are telecentric: the size of an object is not dependent on their position within the field of view.
What does ISIIS-DPI stand for?
ISIIS (In-Situ Ichthyoplankton Imaging System) was first developed to image large volumes of water to survey ichthyoplankton patchiness in the Gulf Stream off the coast of Florida, back in 2005. Since the imager strength is its ability to image over a large depth of field, and since it is capable of imaging more than plankton, we added DPI to its name, for Deep-focus Particle Imager. ISIIS is still the most referred name in published literature.
What kind of camera do you use?
We use industrial cameras, with either a USB3 or GigE interface. A line-scan camera is ideal for application with constant speed in the 5 knots range. An area-scan camera allows the user to deploy at highly variable tow speeds, do vertical profiles or stationary set-ups like moorings.
What is the main advantage of a line-scan camera or area-scan camera?
A line scan camera takes a continuous image and therefore you need not worry about overlapping images (counting taxa twice for example) or sub-sampling. The line-scan camera also allows us to use the entire enclosure viewport diameter as opposed to fitting a “square image” inside a viewport. If you match the scanning rate of the camera with the water speed flowing in between the pod you obtain a true image, neither stretched nor compressed. An area-scan camera may be more versatile and easier to use.
What about motion blur?
The light enclosure shines lots of light onto the camera, and therefore we are always very close to the smallest exposure time allowed by the camera sensor. Motion blur has never been an issue thus far.
Can you explain pixel-resolution?
Pixel resolution is a function of the number of pixels available on the camera sensor and the size of the image projected onto that sensor. A sensor with 2440 pixels across the width and 2048 pixels high has a resolution of 2048x2440. If the projected image is 12cm high, each pixel of the sensor captures 120,000um / 2048 = 58.6um / pixel. You need at least 10 to 15 pixels to start representing an object adequately . Such a camera resolution would therefore be acceptable to capture taxa that is about 700um in size or larger
What about depth of field?
The pixel resolution, as explained above, is found to correlate very well with the 1951 USAF resolution test chart and test device originally defined by the U.S Air Force, at the focal plane of the shadowgraph. As targets move away from this optimal focus plane (moving closer to the camera pod viewport or the light projector viewport), they will become more and mmore blurry. Most users will set the imager so its resolution stays within a factor of 2 or 3. For example, if the best resolution, at the focal plane, is 50um/pixel, the depth of field will be set so resolution does not get worse than 100 to 150um/pixel. We found that identification of organisms larger than 1 mm can be done over 30cm depth of field. However, if your interest is in smaller taxa, in the 300 to 500μm range, you will probably limit the depth of field to be about 10 to 15cm wide. Taxa in the 50um to 100um will dictate a DOF of 1cm or less.
What about imaging different size taxa altogether?
To tackle this issue, several groups have used two imagers side by side. One imager would focus on smaller organisms over a shallow depth of field while the other would image much larger taxa over a larger depth of field. Another approach has been to stack imagers. For example, stacking high resolution shadowgraph on top of each other allows to capture large tax while maintaining a high resolution.
How do I calculate the volume of water imaged per second?
This comes down to simple maths: Total area imaged by the camera x Depth of Field distance x Number of image per second Larger ISIIS-DPI systems are capable of imaging up to 80L/sec per camera
PUBLICATIONS
The ISIIS-DPI technology has been cited in over 238 publications globally (Google Scholar search) and it has been quoted to "have changed the way we do science"
-
Cowen RK, Greer AT, Guigand CM, Hare JA, Richardson DE, Walsh HJ (2013) Evaluaon of the in situ ichthyoplankton imaging system (ISIIS): Comparison with the tradional (bongo net) sampler. Fish Bull 111:1-12
-
Dzwonkowski B, Greer AT, Briseño-Avena C, Krause JW, Soto IM, Hernandez FJ, Deary AL, Wiggert JD, Joung D, Fitzpatrick PJ, and others (2017) Estuarine influence on biogeochemical properes of the Alabama shelf during the fall season. Cont Shelf Res 140:96-109
-
Dzwonkowski B, Fournier S, Reager JT, Milroy S, Park K, Shiller AM, Greer AT, Soto I, Dykstra SL, Sanial V (2018) Tracking sea surface salinity and dissolved oxygen on a river-influenced, seasonally strafied shelf, Mississippi Bight, northern Gulf of Mexico. Cont Shelf Res 169:25-33
-
Failleaz R, Picheral M, Luo JY, Guigand C, Cowen RK, Irisson J (2016) Imperfect automac image classificaon successfully describes plankton distribuon paerns. Methods in Oceanography 15-16:60-77
-
Greer AT (2018) In-situ shadowgraph imaging. Mar Technol Soc J 52:62-65
-
Greer AT, Boyee AD, Cruz VJ, Cambazoglu MK, Dzwonkowski B, Chiaverano LM, Dykstra SL, Briseno-Avena C, Cowen RK, Wiggert JD (in press) Contrasng fine-scale distribuonal paerns of zooplankton driven by the formaon of a diatom-dominated thin layer. Limnol Oceanogr
-
Greer AT, Briseno-Avena C, Deary AL, Cowen RK, Hernandez FJ, Graham WM (2017) Associaons between lobster phyllosoma and gelanous zooplankton in relaon to oceanographic properes in the northern Gulf of Mexico. Fisheries Oceanography 26:693-704
-
Greer AT, Cowen RK, Guigand CM, McManus MA, Sevadjian JC, Timmerman AHV (2013) Relaonships between phytoplankton thin layers and the fine-scale vercal distribuons of two trophic levels of zooplankton. J Plankton Res 35:939-956
-
Greer AT, Shiller AM, Hofmann EE, Wiggert JD, Warner SJ, Parra SM, Pan C, Book JW, Joung D, Dykstra S, and others (2018) Funconing of coastal river-dominated ecosystems and implicaons for oil spill response: From observaons to mechanisms and models. Oceanography 31:90-103
-
Greer AT and Woodson CB (2016) Applicaon of a predator – prey overlap metric to determine the impact of sub-grid scale feeding dynamics on ecosystem producvity. ICES J Mar Sci 73:1051-1061
-
Greer AT, Chiaverano LM, Diy JG, Hernandez FJ (2019) In situ observaons of fish larvae encased within a pelagic gelanous matrix. Mar Ecol Prog Ser 614:209-214
-
Greer AT, Woodson CB, Guigand CM, Cowen RK (2016) Larval fishes ulize Batesian mimicry as a survival strategy in the plankton. Mar Ecol Prog Ser 551:1-12
-
Greer AT, Cowen RK, Guigand CM, Hare JA (2015) Fine-scale planktonic habitat paroning at a shelf-slope front revealed by a high-resoluon imaging system. J Mar Syst 142:111-125
-
Greer AT, Chiaverano LM, Luo JY, Cowen RK, Graham WM (2018) Ecology and behaviour of holoplanktonic scyphomedusae and their interacons with larval and juvenile fishes in the northern Gulf of Mexico. ICES J Mar Sci 75:751-763
-
Greer AT, Woodson CB, Smith CE, Guigand CM, Cowen RK (2016) Examining mesozooplankton patch structure and its implicaons for trophic interacons in the northern Gulf of Mexico. J Plankton Res 38:1115-1134
-
Greer AT, Cowen RK, Guigand CM, Hare JA, Tang D (2014) The role of internal waves in larval fish interacons with potenal predators and prey. Prog Oceanogr 127:47-61
-
Luo JY, Irisson JO, Graham B, Guigand C, Sarafraz A, Mader C, Cowen RK (2018) Automated plankton image analysis using convoluonal neural networks. Limnol Oceanogr-Meth 16:814-827
-
Luo JY, Grassian B, Tang D, Irisson JO, Greer AT, Guigand CM, McClatchie S, Cowen RK (2014) Environmental drivers of the fine-scale distribuon of a gelanous zooplankton community across a mesoscale front. Mar Ecol Prog Ser 510:129-149
-
McClatchie S, Cowen R, Nieto K, Greer A, Luo JY, Guigand C, Demer D, Griffith D, Rudnick D (2012) Resoluon of fine biological structure including small narcomedusae across a front in the southern California Bight. Journal of Geophysical Research C: Oceans 117
-
Parra SM, Greer AT, Book JW, Deary AL, Soto IM, Culpepper C, Hernandez FJ, Miles TN (2019) Acousc detecon of zooplankton diel vercal migraon behaviors on the northern Gulf of Mexico shelf. Limnol Oceanogr 64:2092-2113
-
Robinson KL, Luo JY, Sponaugle S, Guigand CM, Cowen RK (2017) A tale of two crowds: Public engagement in plankton classificaon. Froners in Marine Science 4:82
-
Schmid MS, Cowen RK, Robinson K, Luo JY, Briseño-Avena C, Sponaugle S (2020) Prey and predator overlap at the edge of a mesoscale eddy: Fine-scale, in-situ distribuons to inform our understanding of oceanographic processes. Sci Rep 10:921
-
Sevadjian JC, McManus MA, Ryan J, Greer AT, Cowen RK, Woodson CB (2014) Across-shore variability in plankton layering and abundance associated with physical forcing in Monterey Bay, California. Cont Shelf Res 72:138-151
-
Timmerman AHV, McManus MA, Cheriton OM, Cowen RK, Greer AT, Kudela RM, Ruenberg K, Sevadjian J (2014) Hidden thin layers of toxic diatoms in a coastal bay. Deep-Sea Res Pt II 101:129-140
-
Moritz S. Schmid, Dominic Daprano, Malhar M. Damle, Christopher M. Sullivan, Su Sponaugle, Charles Cousin, Cedric Guigand and Robert K. CowenEdge computing at sea: high-throughput classification of in-situ plankton imagery for adaptive sampling, Front. Mar. Sci., 08 June 2023, Sec. Ocean Observation, Volume 10 - 2023
